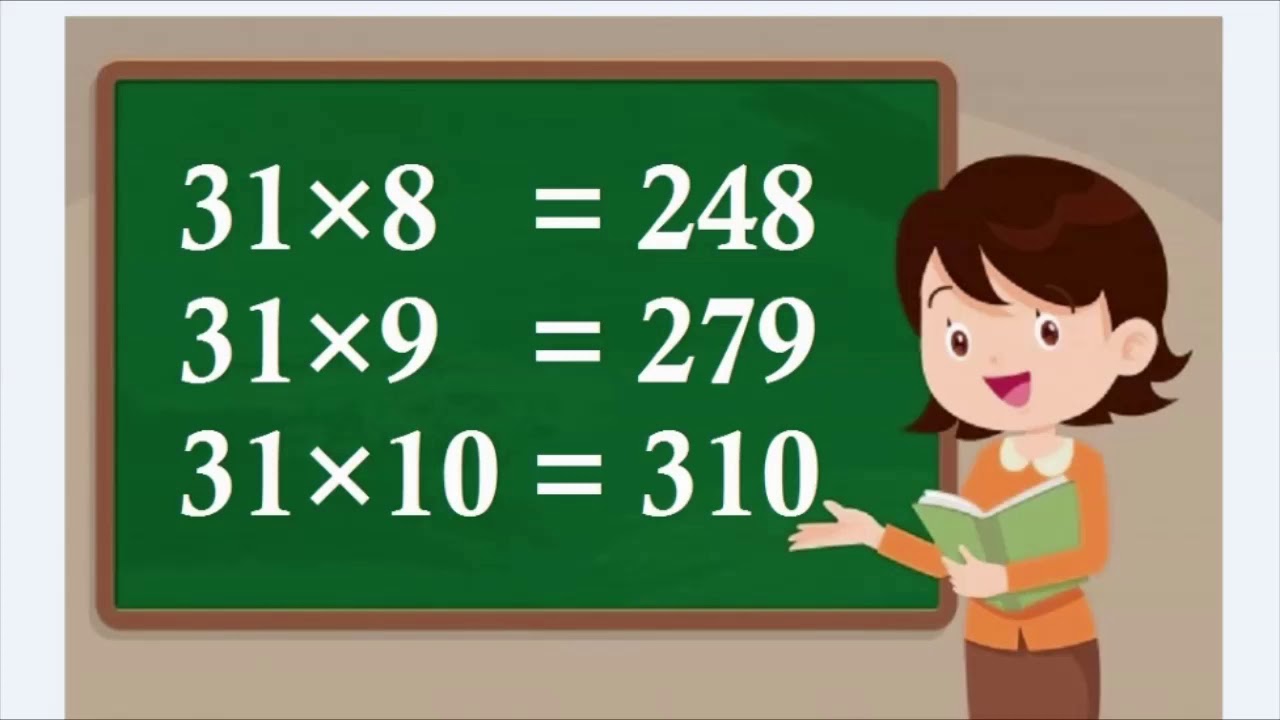
Table of 31, table of thirty one, thirty one ka table, 31 ka table YouTube
The table lists the K a values and the strength of each acid and base. Strong acids are listed at the top left-hand corner of the table and have Ka values >1 Acids with a K a value less than one are considered weak and get weaker as we move to the bottom of the table.

2 ka table YouTube
Strong bases completely dissociate in aq solution (Kb > 1, pKb < 1). Conjugate acids (cations) of strong bases are ineffective bases. * Compiled from Appendix 5 Chem 1A, B, C Lab Manual and Zumdahl 6
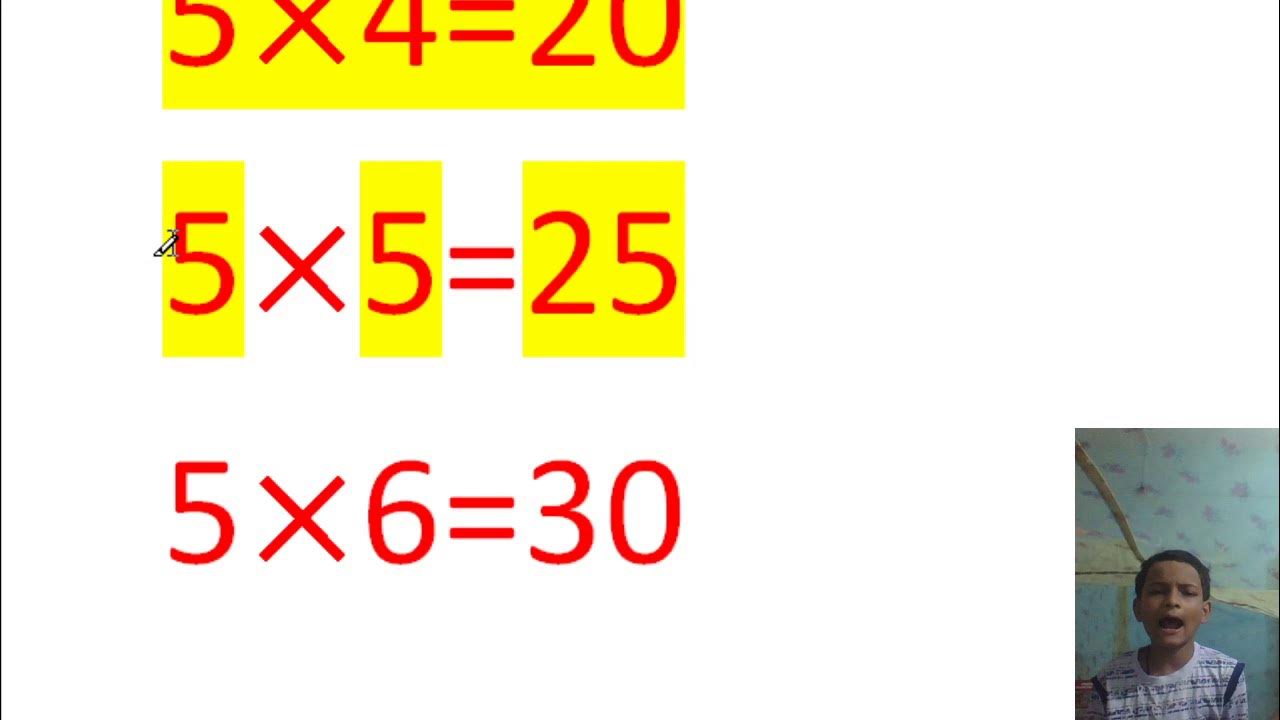
FIFTH KA TABLE YouTube
Acid Base Conjugate Pairs. We will use K (a or b) to represent the acid or base equilibrium constant and K' (b or a) to represent the equilibrium constant of the conjugate pair. For an Acid Base Conjugate Pair. KaKb′ = Kw (16.3.9) (16.3.9) K a K b ′ = K w. Consider the generic acid HA which has the reaction and equilibrium constant of.

Best Tricks To Learn Table Of 31 Up To 20 Times With Pdf 31 Ka Table Shikshaportal
Table of 31 is the representation of repeated addition of the whole number, 31, to itself. For example, 31 multiplied by 3, represents 31 is added three times to itself. 31 x 3 ⇒ 31 + 31 + 31 ⇒ 93 In the same way, we can write the multiplication table of 31 for n natural numbers. Check Tables from 1 to 100 to learn all the tables.

12 ka table YouTube
Today we will tell you some easy methods to memorise it so for this you have to read this post till the end, but first let us see the table of 31 upto 10 and then we will also read it upto 50 later in this post. 31 x 1 = 31. 31 x 2 = 62. 31 x 3 = 93. 31 x 4 = 124. 31 x 5 = 155. 31 x 6 = 186.

19 ka table YouTube
The values of \(pK_a\) and \(pK_b\) are given for several common acids and bases in Table 16.5.1 and Table 16.5.2, respectively, and a more extensive set of data is provided in Tables E1 and E2. Because of the use of negative logarithms, smaller values of \(pK_a\) correspond to larger acid ionization constants and hence stronger acids.

2 ka table YouTube
The larger the Ka, the stronger the acid and the higher the H + concentration at equilibrium. Like all equilibrium constants, acid-base ionization constants are actually measured in terms of the activities of H + or OH −, thus making them unitless. The values of Ka for a number of common acids are given in Table 16.4.1.
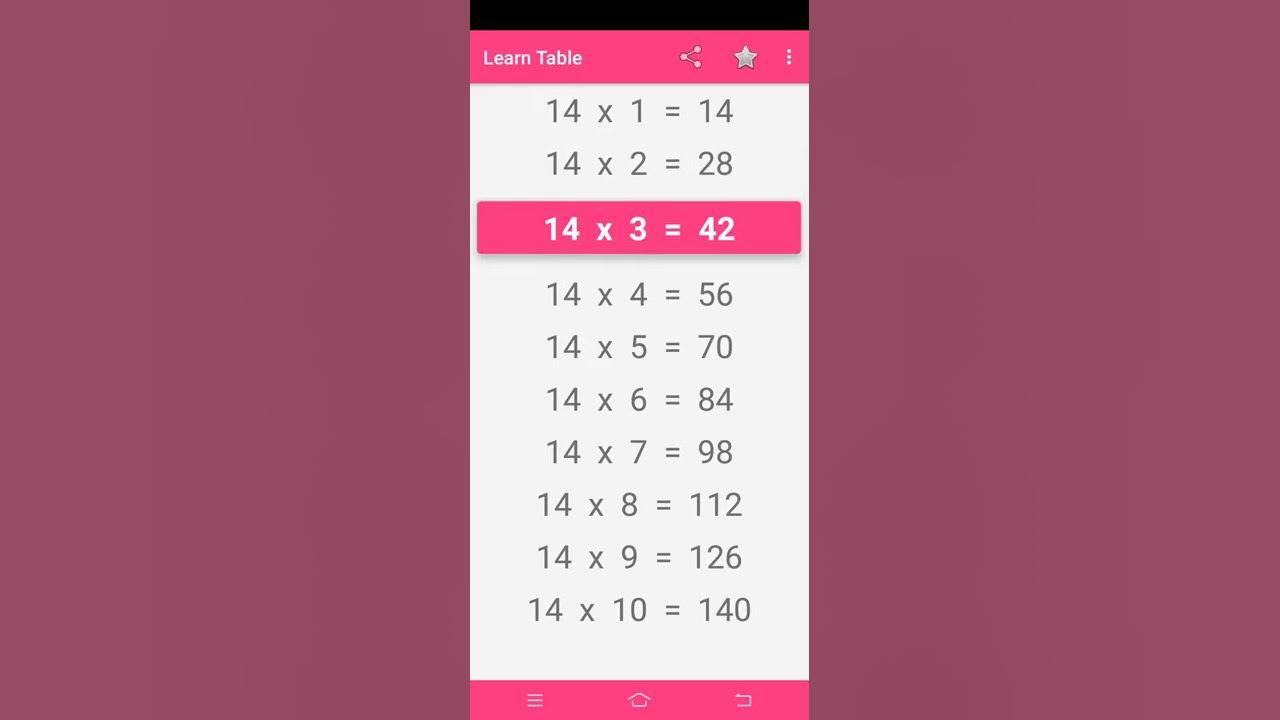
14 ka table YouTube
Table of 31, table of thirty one, thirty one ka table, 31 ka tableआज हमलोग इस विडियों मे देखेगे, पढ़ेगे और याद.

How to make 33 ka table and information ka table
31 ka table with very easy trick| Table of 31| 31 ka table| #table_trick #shorts #mersh_classes

31 ka Pahada Hindi mein Multiplication table 31 ka Table Table of 31 Maths table YouTube
table of 31,31 ka table,table of 31 32 33,multiplication table of 31,table,31 table,table of thirty one,what is the table of 31,table 31 to 40,tables,31 tabl.
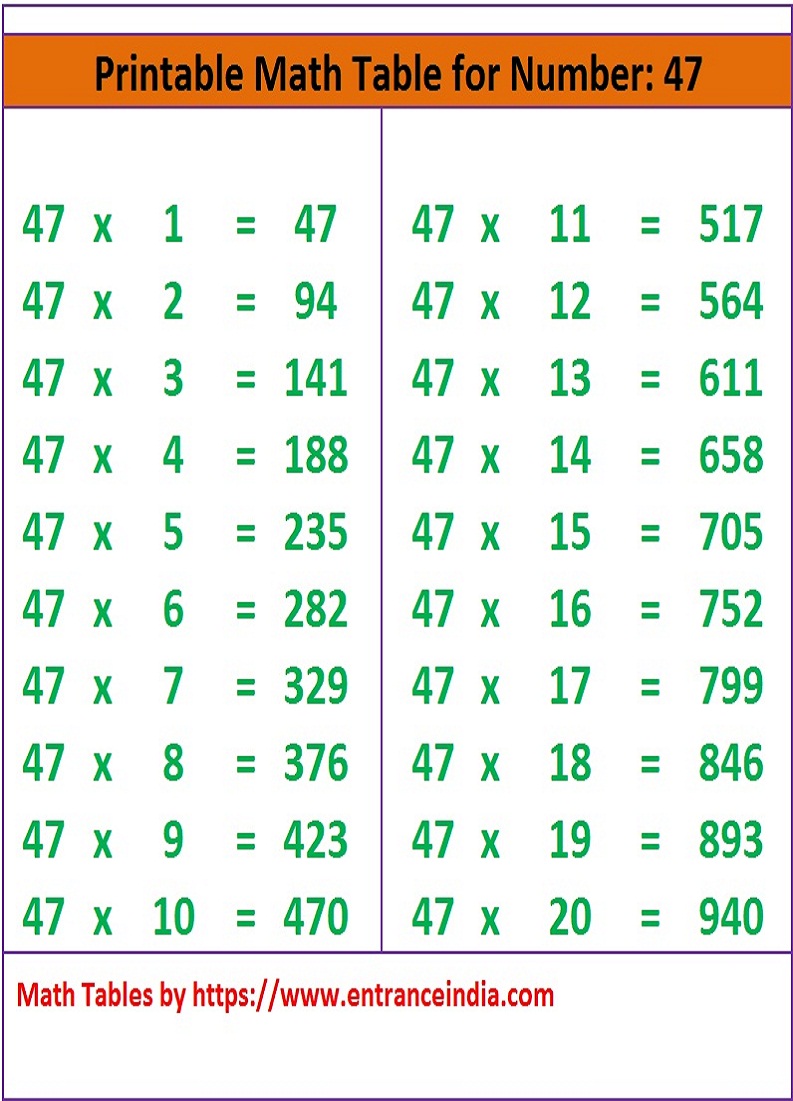
Math Table Printable Downloadable for 47 ka Pahada ENTRANCE INDIA
1. Strong acids are listed at the top left hand corner of the table and have Ka values >1 2. Acid with values less than one are considered weak. 3. The strong bases are listed at the bottom right of the table and get weaker as we move to the top of the table.
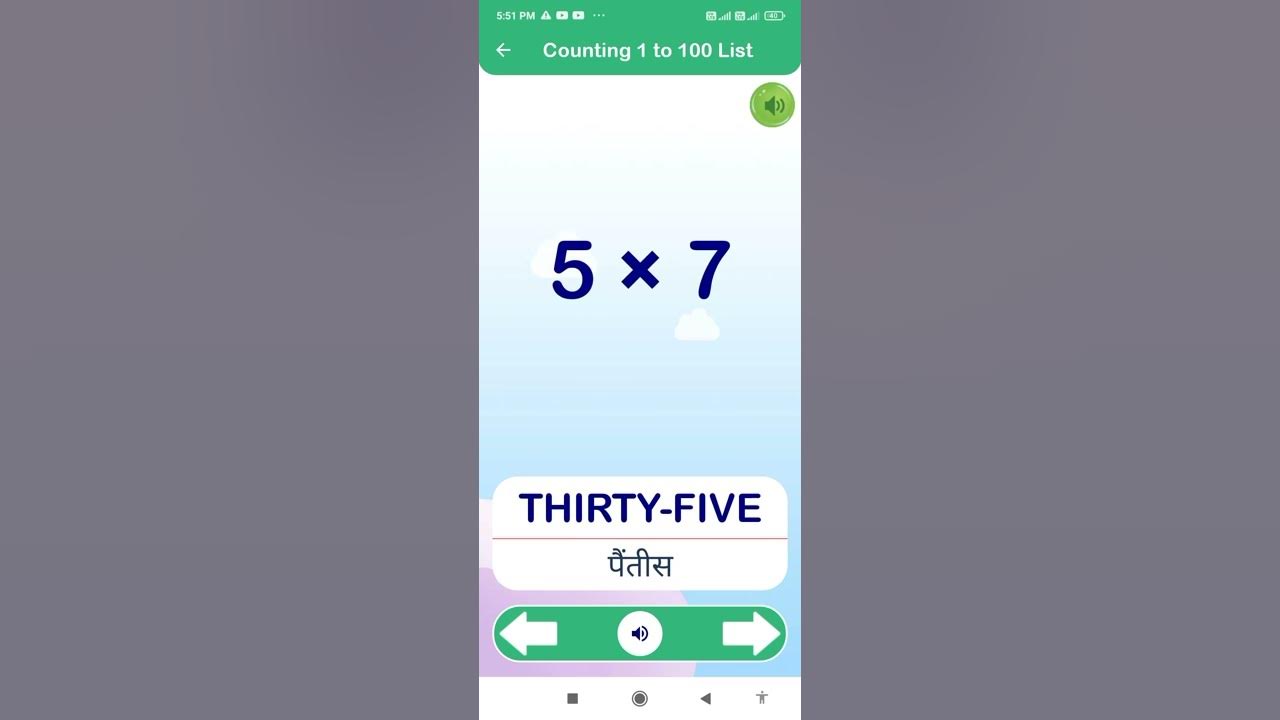
5 ka table YouTube
Updated on February 03, 2020 K a is the equilibrium constant for the dissociation reaction of a weak acid. A weak acid is one that only partially dissociates in water or an aqueous solution. The value of K a is used to calculate the pH of weak acids. The pK a value is used to choose a buffer when needed.
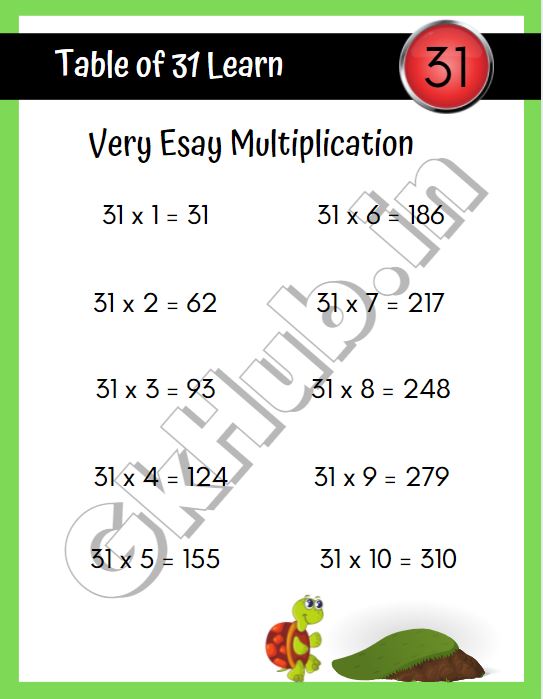
31 का पहाड़ा 31 Ka Pahada 31 ka Table in Hindi GK Hub
Ka Table. 1.5×10-10. Urea hydrogen ion NHCONH6.7×10-1. Zinc 2+ ion Zn2+2.5×10-10.

9 ka table YouTube
Looking at Table 5.2.1 5.2. 1, you see that the pK a of carboxylic acids are in the 4-5 range, the pK a of sulfuric acid is -10, and the pK a of water is 14. Alkenes and alkanes, which are not acidic at all, have pK a values above 30. The lower the pKa value, the stronger the acid. Table 5.2.1 5.2. 1: Representative acid constants.

Table Of 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 Brainly.in 633 in 2022
Successive acid dissociation constants are provided for polyprotic weak acids; where there is ambiguity, the specific acidic proton is identified. To find the Kb value for a conjugate weak base, recall that. Ka ×Kb = Kw (E5.1) (E5.1) K a × K b = K w. for a conjugate weak acid, HA, and its conjugate weak base, A -. Compound.
17 ka table YouTube
pKa is an acid dissociation constant used to describe the acidity of a particular molecule. Its value is directly related to the structure of the given compound. The constant changes depending on the solvent the compound is used in. Typically, organic chemists compare the various values from their determination in water, DMSO and the gas phase.